Our approach
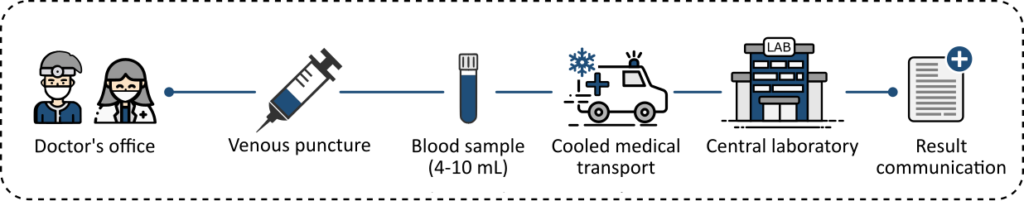
Centralized strategy
IVD technologies have revolutionized healthcare but because they still depend on a laboratory environment for quantitative results, they remain bound to the traditional centralized healthcare approach.
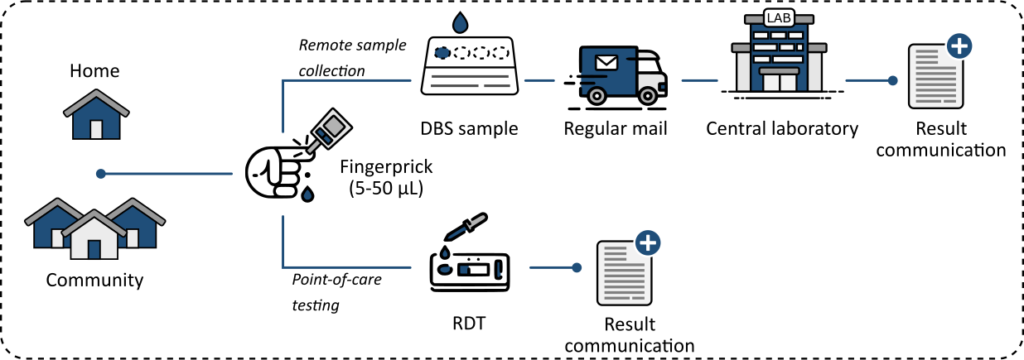
Decentralized strategy
However, there is a mounting body of evidence that this practice does not address the current worldwide trends and expectations on how to manage and treat diseases. This has resulted in the growing urge to move towards a decentralized approach, expecting to bring time- and cost-benefit for patients, doctors, and the healthcare system in general. Over the past decade, we have witnessed enormous efforts in the IVD field to bring lab-quality bioassays from the lab to the POC (i.e., clinics, homes, and remote communities).
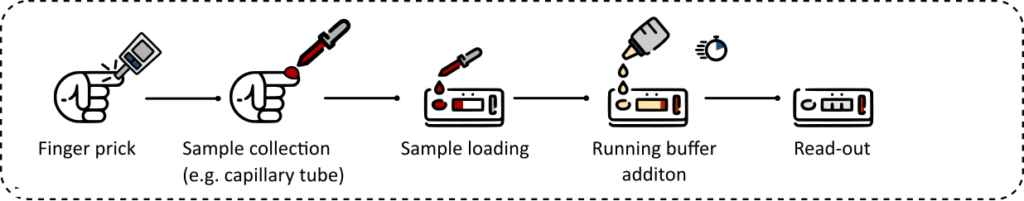
Traditional RDT
While lab-on-a-chip (LoC) technologies, enabling complex fluid manipulations for miniaturized and automated bioassays on a chip, have long promised to disrupt the IVD market, clinically relevant solutions have so far failed to make a significant impact and reach the market. This can be attributed to the complexity of integration/operation, high cost, off-chip sample preparation, to name a few. However, some qualitative RDTs have reached the market but they still require manual off-chip pre-processing of sample (e.g., metering or dilution). This limits their usage to only trained and experienced operators, therefore preventing their broad implementation as POC devices. All these shortcomings were in the spotlight during the COVID-19 pandemic, which has been perceived as a real game-changer in the field of IVD POC. While this pandemic provided many medical device developers with the opportunity to test their novel POC systems in the field already in an early phase, it also confronted them with the many access and practical issues encountered from the end-user side.
Hence, the DECIPHER consortium aims to revolutionize the IVD POC field by developing for the first time a true quantitative sample-to-result POC test. This will be achieved by building a DECIPHER patch capable of:
- Biofluids (self-)sampling via HMNs
- Immediate analysis of this sample on the very same patch
Molecular bioassays
Quantitative readout
High-throughput microfabrication
AI-based socio-economic system analysis
Life cycle assessment
DECIPHER sampling module
The sampling module will initially be validated using benchtop models, such as artificial tissues, followed by using in vivo animal models and ultimately performing blood sampling trials on healthy volunteers.
DECIPHER bioassay modules
The bioassay module will be validated with both biobanked plasma samples (retrospective clinical validation) as well as with blood collected from patients (prospective clinical validation), next to validation of the DECIPHER patch in a controlled clinical setting.
